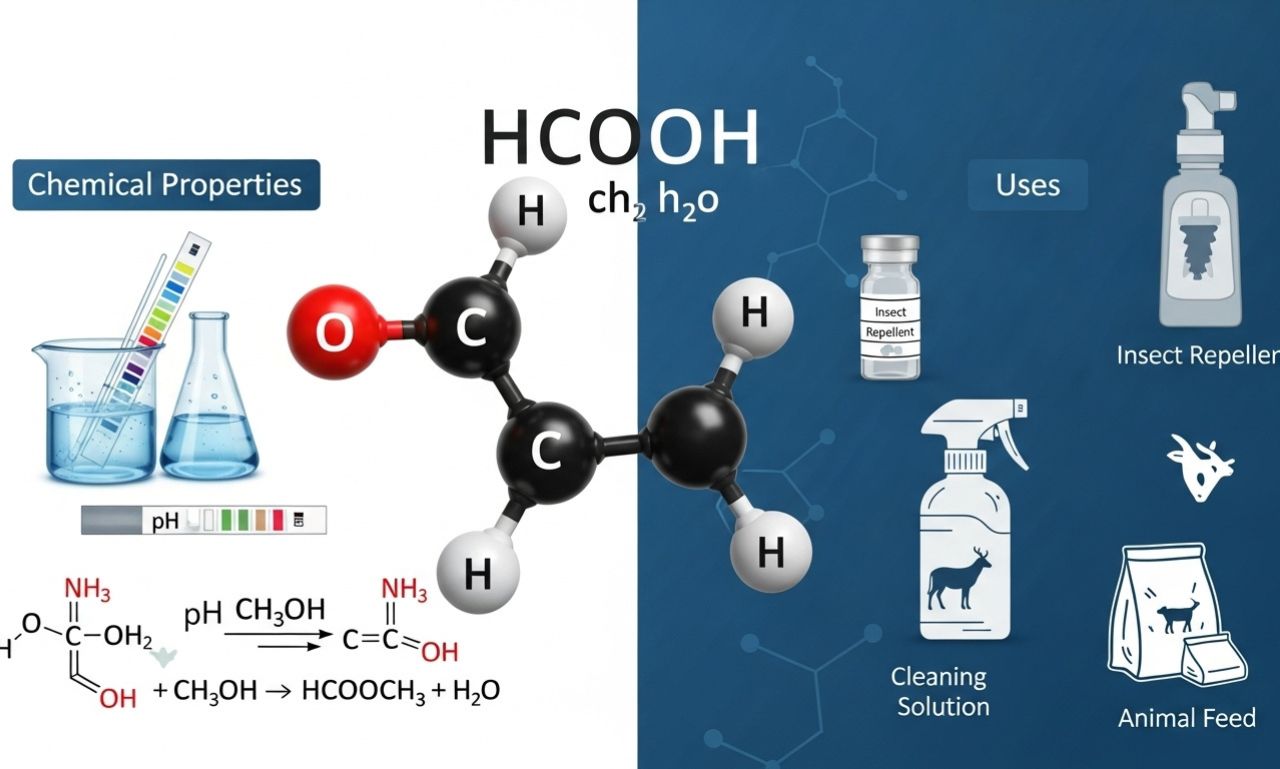
The notation hcooch ch2 h2o appears in some chemical discussions, yet it often creates confusion because it doesn’t correspond to a single, officially recognized compound. Instead, it seems to describe a system or reaction mixture where a formate ester (or formic-acid-derived unit), a methylene (CH₂) fragment, and water (H₂O) interact.
This article unpacks what each part means, how the combination behaves, and what practical implications or scientific value it holds. You’ll also see why this formula became popular online and what it really represents in chemistry.
The component: Formate or HCOO- (from Formic Acid)
Formic acid (HCOOH) is the simplest carboxylic acid. It’s a colorless, fuming liquid that occurs naturally in ant venom and some plants. It is a versatile chemical, acting as both a reducing agent and an acid.
In the context of hcooch ch2 h2o, the “HCOO” portion could represent a formate ester, such as methyl formate (HCOOCH₃), or simply the formic acid group itself. Chemically, formates are highly reactive in the presence of water and catalysts, which makes them useful in synthesis and fuel production.
Formic acid has a relatively strong acidity and good solubility in water and alcohols. It can decompose under heat or catalysis, releasing hydrogen and carbon dioxide — a property that’s particularly useful in hydrogen fuel technology.
The component: CH₂ (Methylene Fragment)
The methylene group (–CH₂–) is one of the most important structural elements in organic chemistry. It forms the backbone of countless molecules, from hydrocarbons to polymers.
In the hcooch ch2 h2o notation, the CH₂ fragment might stand for a methylene bridge connecting two larger structures, or it may symbolize part of an intermediate species like formaldehyde or methanol. Methylene itself, as a free carbene (:CH₂), is unstable and highly reactive, but within molecules, it stabilizes as a linking unit that can easily participate in addition or substitution reactions.
Thus, CH₂ provides the organic “bridge” between formate and water chemistry, contributing to reaction diversity.
The component: H₂O (Water)
Water is the universal solvent — essential in nearly all chemical reactions. In the hcooch ch2 h2o system, it performs multiple roles:
-
It acts as a solvent, allowing other components to interact.
-
It serves as a reactant in hydrolysis reactions, breaking ester bonds to yield acids and alcohols.
-
It stabilizes ionic intermediates through hydrogen bonding and solvation.
In essence, water ensures that the reaction environment for hcooch ch2 h2o remains dynamic and reactive.
What Exactly Does hcooch ch2 h2o Represent?
Because there’s no single known compound with this exact formula, chemists interpret it in several ways:
-
It might represent a reaction mixture involving a formate ester reacting with water and a CH₂-based intermediate.
-
It could symbolize the hydrolysis of methyl formate (HCOOCH₃) with water to form formic acid (HCOOH) and methanol (CH₃OH).
-
Some consider it a shorthand for broader aqueous formate chemistry rather than an individual substance.
In short, hcooch ch2 h2o isn’t a single molecule but a descriptor of a chemical environment or process.
Reaction Pathways Involving hcooch ch2 h2o
Esterification and Hydrolysis
The most direct reaction represented by this system is the hydrolysis of a formate ester.
Example:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
Here, water breaks the ester bond to produce formic acid and methanol. The methanol molecule contains the CH₂ group implied in the notation. This process is fundamental in both laboratory and industrial chemistry.
Polymerization and Methylene Insertion
The CH₂ component might also refer to reactions where methylene units link molecules, forming polymers or resins. When formate or other carboxylic groups are present, they can act as linkers or side chains in polymer synthesis.
Catalytic Redox Processes
Formic acid and formates are important in hydrogen generation and catalytic reduction. In aqueous environments, these substances can decompose into CO₂ and H₂, making the system useful for clean-energy research.
Properties of the hcooch ch2 h2o System
Even though it isn’t a single compound, we can still describe typical characteristics of the system’s components:
-
Solubility: Excellent in water due to the polar nature of both formate and methanol.
-
Reactivity: High reactivity in acidic or basic conditions; prone to hydrolysis or decomposition.
-
Polarity: Strongly polar, making it suitable for polar reactions and catalytic processes.
-
Thermal Stability: Formate systems decompose at high temperatures, sometimes releasing hydrogen gas.
These attributes make hcooch ch2 h2o-type systems both reactive and useful in controlled settings.
Industrial and Laboratory Applications of hcooch ch2 h2o
Even though the formula isn’t officially recognized, its underlying chemistry finds numerous applications:
-
Organic Synthesis: Formate esters are key intermediates in the production of pharmaceuticals and fine chemicals. Their hydrolysis and esterification reactions are well understood and widely utilized.
-
Fuel and Energy Storage: Formic acid acts as a hydrogen carrier, providing a safer, denser alternative to gaseous hydrogen for fuel cells.
-
Polymer and Resin Production: Methylene bridges and formate groups can combine in reactions leading to biodegradable polymers.
-
Green Chemistry: Systems combining water, simple esters, and CH₂ fragments are environmentally friendly and align with sustainable chemical manufacturing.
Thus, while hcooch ch2 h2o may be informal, its chemistry contributes to real industrial progress.
Safety and Handling Considerations
Working with hcooch ch2 h2o-type mixtures requires awareness of potential hazards:
-
Formic acid is corrosive and can cause burns on skin contact.
-
Esters like methyl formate are flammable and may emit irritating vapors.
-
Proper ventilation and personal protective equipment (gloves, goggles, lab coats) are essential.
-
Temperature control helps prevent unwanted decomposition or vaporization.
-
Waste disposal must follow chemical-safety regulations to avoid environmental contamination.
Treating these components with care ensures both lab safety and process integrity.
Common Misconceptions About hcooch ch2 h2o
-
It’s a compound – False. There’s no molecule with that exact formula registered in chemical databases.
-
It’s dangerous by itself – Not necessarily; safety depends on which real chemicals are in the mixture.
-
It’s newly discovered – Incorrect. The chemistry of formates, methylene, and water has been studied for decades.
-
It’s only theoretical – Some online discussions treat it as fiction, but its core chemistry is well-established in practical applications.
Understanding what hcooch ch2 h2o actually represents helps dispel confusion and encourages correct scientific usage.
The Role of hcooch ch2 h2o in Green Chemistry
Modern chemistry emphasizes sustainability, and this system aligns well with those goals. Water acts as a green solvent, and formate esters can be derived from renewable carbon sources. Additionally, the decomposition of formic acid to release hydrogen supports clean energy production.
When combined with methylene-based linkers, such systems allow for biodegradable materials and lower toxicity — hallmarks of eco-friendly design.
Research and Future Outlook
Scientists are increasingly interested in aqueous formate systems for energy and synthetic chemistry. Current research focuses on:
-
Hydrogen storage technologies, using formate salts as efficient hydrogen donors.
-
Catalyst development, improving reaction rates in water-based media.
-
Biodegradable polymers, incorporating CH₂ bridges and formate functional groups.
-
Astrochemical detection, as formate esters like methyl formate have been observed in interstellar clouds.
Future work will likely refine these processes, improving efficiency, safety, and sustainability.
Why Understanding hcooch ch2 h2o Matters
Learning about this notation helps chemists, students, and industry professionals understand broader chemical principles: reaction mechanisms, hydrolysis, redox behavior, and molecular design.
It also emphasizes the importance of clear chemical communication. Ambiguous notations like hcooch ch2 h2o remind us that while chemistry is universal, clarity in writing is essential for accurate understanding.
Frequently Asked Questions
What is hcooch ch2 h2o?
It’s not a single compound but a shorthand description for systems combining formate esters, methylene fragments, and water.
Is hcooch ch2 h2o used in laboratories?
Yes, indirectly. The underlying reactions involving formate esters and water are common in chemical and industrial labs.
Does hcooch ch2 h2o exist naturally?
Not as a distinct molecule, but its components — formic acid, CH₂ fragments, and water — are all found in nature.
Can hcooch ch2 h2o be hazardous?
It depends on the actual materials present. Formic acid is corrosive, while methyl formate is flammable, so caution is needed.
What reactions involve hcooch ch2 h2o?
Typical reactions include hydrolysis of esters, decomposition to generate hydrogen, and polymerization involving CH₂ linkers.
Why is the formula sometimes written differently?
Because it’s not standardized, writers sometimes use “HCOOCH CH₂ H₂O” or “HCOOCH₃ + H₂O” to express similar concepts.
Conclusion
The term hcooch ch2 h2o might look mysterious, but it’s simply a symbolic way to describe the interaction between formate esters, methylene fragments, and water. Although no compound by that exact name exists, the chemistry it reflects — hydrolysis, catalysis, and hydrogen production — is vital to both modern industry and green technology.
By understanding each component and its role, we uncover valuable insights into sustainable reactions, clean energy, and organic synthesis pathways. hcooch ch2 h2o reminds us that chemistry’s language evolves, but the principles remain grounded in real, observable science.