The term DGH A has begun appearing in professional and technical circles — especially in ophthalmology and digital-device sectors — often referring to a portable biometry ultrasound system. In this article, we’ll explore what DGH A stands for, how it works, its applications, benefits, limitations, and why it matters now more than ever. We’ll also consider future trends and FAQs.
Using transition words such as “however”, “moreover”, and “on the other hand”, we’ll make sure the discussion flows smoothly and remains reader-friendly.
What is DGH A?
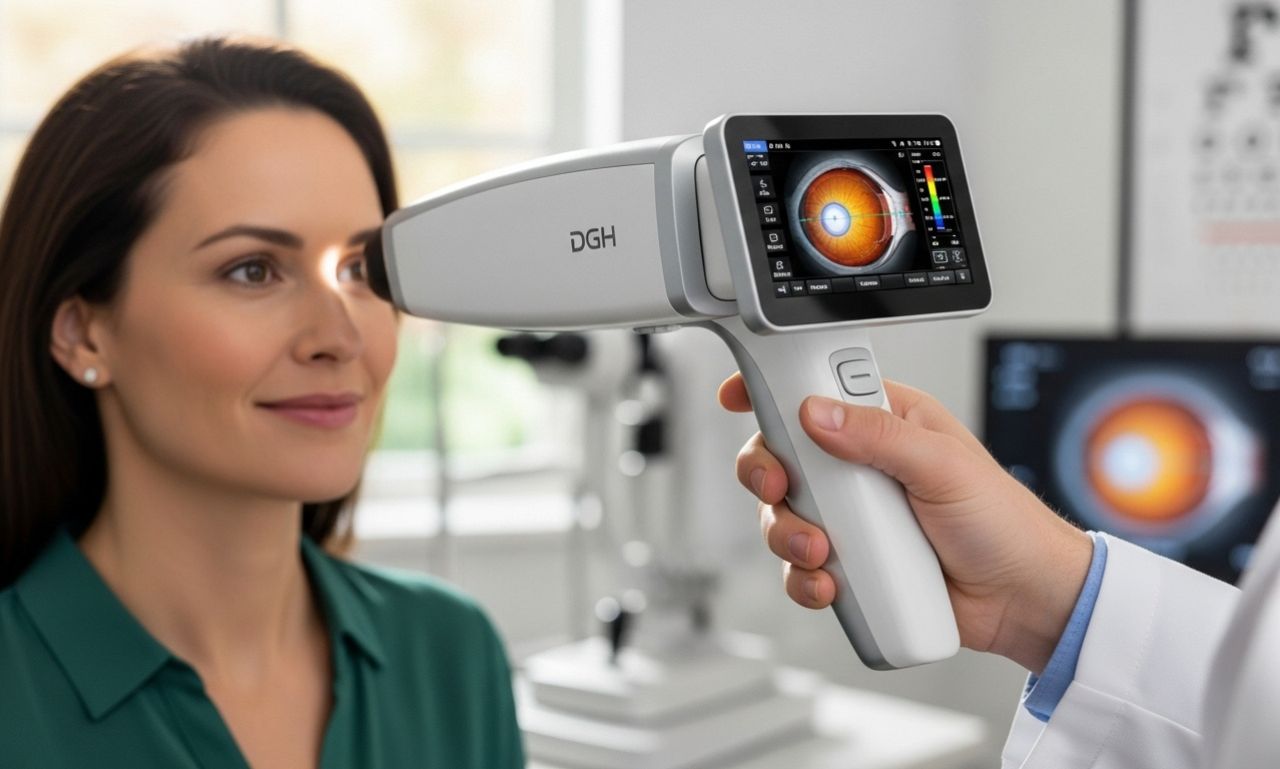
At its core, DGH A is used to refer to the DGH Technology “Scanmate A” (also known as “DGH 6000 A-Scan”) device. It is a compact, USB-connected ocular ultrasound biometer designed for measuring key eye parameters like axial length, anterior chamber depth and lens thickness.
Essentially, DGH A allows eye clinics to perform biometry efficiently by plugging into a laptop, rather than relying on large stationary cabinets or cart-based machines. It features contact or immersion measurement modes, audible alignment feedback and IOL (intraocular lens) calculation tools built in.
Thus, DGH A stands for a device rather than a concept, though the acronym may sometimes appear generically. When you see DGH A in an ophthalmic equipment context, it almost always means this DGH Technology portable A-Scan system.
Historical development of DGH A
The evolution of DGH A began with DGH Technology’s effort to make ocular biometry more accessible and efficient. Traditional A-scan machines required dedicated consoles, bulky hardware and often limited mobility. DGH introduced the Scanmate A (DGH 6000) which shifted the paradigm toward a laptop-based ultrasound probe and software suite.
Over time, enhancements such as improved alignment-feedback (e.g., 1- to 3-star rankings for probe alignment), compression lock-out features for contact measurements, immersion shell compatibility and modern IOL formulas were incorporated.
More recently, clinics report faster workflow, better data integration (EMR/EHR compatibility), and portability advantages. These developments make DGH A a significant tool for modern ophthalmic practices.
Technical features of DGH A
Key features of DGH A include:
-
Probe frequency: 10 MHz single-element transducer for axial measurements.
-
Measurement modes: Contact mode (direct probe to cornea) or immersion mode (using a Prager Shell® water bath to avoid corneal compression).
-
Axial length range: Approximately 15.00 mm to 40.00 mm.
-
Software built-in IOL formulas: SRK II, SRK/T, Hoffer Q, Haigis, Holladay I, Binkhorst II, and post-refractive formulas.
-
Data management: Patient database searchable, backup tool, print/PDF report export, EMR/EHR compatibility.
-
Portability: USB interface plugs into standard Windows computer, compact form factor (5.75″ × 3.5″ for the module) and carrying case.
These features make the DGH A a modern biometry tool that balances precision, flexibility, and integration.
Applications of DGH A in practice
In practical terms, DGH A is used in:
-
Preoperative cataract screening: accurate axial length and lens thickness measurements are essential for selecting correct intraocular lens (IOL) power.
-
Myopia management and axial length progression monitoring: some clinics use the axial-length progression reports built into the software.
-
Outreach or mobile clinics: because of the portable nature, DGH A enables setting up screening in satellite clinics, community centres or remote sites.
-
Clinics with limited space or needing flexible measurement setups: instead of a fixed large machine, the USB-plug device can be moved between rooms.
-
Research settings: the data export and high measurement reproducibility make it useful for clinical studies.
Benefits of DGH A
The benefits of DGH A are significant:
-
Speed: Because the software provides real-time feedback (audible tones, alignment star system), fewer repeat measurements are needed; this reduces patient time and staff resource.
-
Portability: Being USB-powered and laptop-compatible means the device can be mobilised; useful for outreach, temporary setups or small clinics.
-
Data integration: With exportable reports and EMR-compatibility, data errors are reduced, and administrative workflow improves.
-
Accuracy and reliability: The immersion mode option and alignment feedback help ensure better measurement consistency, reducing error due to corneal compression or misalignment.
-
Cost-effectiveness: While initial cost may still be significant, the reduced infrastructure footprint compared to larger systems, plus potential time savings, make the investment more favourable in many settings.
-
Future-ready: With modern IOL formulas and software updates, it supports evolving clinical practice rather than being static.
Limitations and considerations
However, there are also caveats to consider when adopting DGH A:
-
Operator dependence: As with most ultrasound measurements, user technique still matters (probe alignment, contact pressure, immersion setup). The audible feedback helps, but training remains essential.
-
Environment requirements: Immersion mode, while more accurate in avoiding corneal compression, requires a water bath setup (Prager shell) and careful handling, which may limit speed or portability in some setups.
-
Compatibility and integration: While the software is EMR/EHR compatible, clinics must ensure their systems support the required drivers, data formats and workflow. Some integration may require IT support.
-
Initial cost: Though more compact, the device still carries a cost (e.g., MSRP around US$4,500 as listed) which may be significant for smaller clinics.
-
Maintenance and calibration: As with all diagnostic devices, periodic calibration, servicing and software updates are required. Without this, measurement accuracy may degrade over time.
-
Limited to certain measurement types: DGH A is aimed at A-scan biometry; if your practice requires B-scan ultrasound, anterior segment imaging, or other modalities, you may need additional equipment (though DGH Technology offers other systems).
By weighing both benefits and limitations, clinics can make informed decisions about the role of DGH A in their practice.
Real-world case studies and feedback
Clinics that have switched to DGH A report measurable improvements. For example, one study indicated that switching to the portable A-scan reduced pre-operative measurement time by around 25%.
Another user testimonial: “We have used DGH pachymeters (from the same company) for five years … simple and easy to use.” While this refers to the pachymeter line, it illustrates the brand’s reputation for usability.
In one outreach scenario, a mobile eye screening unit used the USB device to move between screening tents, demonstrating the advantage of portability in remote settings. This kind of flexibility is harder to achieve with traditional equipment.
Such feedback highlights that beyond the technical specs, the real value lies in workflow improvement and adaptability.
Best practices for implementing DGH A
To maximise the benefits of DGH A, consider the following best practices:
-
Provide training for staff on probe alignment, immersion vs contact modes and software features. This helps minimise measurement errors and repeat scans.
-
Use a standard operating procedure (SOP) for data handling: name conventions, export formats, EMR integration, backups.
-
Schedule routine firmware and software updates to ensure the device remains current and capable of the latest IOL formulas.
-
Perform regular calibration checks to maintain measurement accuracy. Keep logs of maintenance.
-
Maintain a spare unit or backup plan in outreach scenarios to avoid downtime.
-
Track key performance indicators (KPIs) such as measurement time per patient, repeat measurement rate, integration errors, patient throughput pre- and post-deployment.
-
Engage stakeholders early: clinicians, technicians, IT staff and administrators. Their buy-in ensures smoother adoption and realisation of ROI.
By implementing these practices, clinics can ensure DGH A becomes a valuable asset rather than just a new gadget.
The future of DGH A and ocular biometry
Looking ahead, DGH A and similar mobile biometry devices are poised for further evolution. Some of the anticipated trends include:
-
AI-assisted measurement: Automated probe alignment and waveform analysis may reduce operator dependence and further improve measurement speed and accuracy.
-
Cloud connectivity and tele-ophthalmology: Remote screening sites could transmit data directly to central clinics, enabling outreach programmes and scalable services.
-
Subscription models / dongle licensing: Instead of one-time purchase, future models may offer subscription or cloud-based billing, reducing upfront cost burden.
-
Extended measurement modalities: Combining A-scan, B-scan and UBM functionalities in a single portable device may broaden clinical use. Indeed, DGH offers multi-probe systems.
-
Global deployment in low-resource settings: The portability and connectivity of devices like DGH A make them well-suited for eye-care in rural or underserved regions.
Together, these trends indicate that DGH A is not just a standalone device but part of a larger shift toward flexible, data-driven, mobile ophthalmic diagnostics.
Why DGH A matters today
In modern eye-care settings, time, space and data integration are all premium assets. DGH A addresses all three by enabling fast, accurate measurement in a compact form, linking results to digital workflows and enabling mobility.
Moreover, as clinics worldwide face growing demands (aging populations, rising cataract volumes, more refractive surgeries), the ability to optimise pre-operative workflows becomes critical. DGH A helps meet this demand.
And with the broader trend toward tele-medicine and mobile screening camps, devices like DGH A support outreach and scalability — bringing quality diagnostics closer to patients rather than requiring them to come to large fixed centres.
Conclusion
In summary, DGH A stands out as a modern, portable, workflow-friendly biometry device that addresses many of today’s challenges in ophthalmology. By combining precision measurement, compact design and robust software features, it supports both mainstream and outreach settings. While adoption requires training, integration and cost-consideration, the benefits — especially in terms of speed, flexibility and data compatibility — are compelling. As eye-care continues to evolve toward mobile, connected, data-driven models, devices like DGH A will increasingly matter. If your clinic is seeking to modernise its biometry workflow or expand into satellite/mobile screening, DGH A is a solution worth serious consideration.